Rehabilitation medicine
Geriatric medicine
Mechanobiology
Orthopaedics
Systems biology
IIJIMA Hirotaka[Affiliation April 2022-September 2023]
Starting year 2022
Nagoya University
Institute for Advanced Research / Graduate School of Medicine
YLC Designated Assistant Professor
Research Areas:Life Science Informatics
Research fields
Research Interests
Musculoskeletal system
Exercise
Aging
Extracellular matrix
Epigenetics
Professional Memberships
Japanese Physical Therapy Association
The Japanese Society of Cartilage Metabolism
Osteoarthritis Research Society International
Main research topics
Aging is the irreversibly progressive decline of physiological function, which eventually leads to age-related diseases, such as osteoporosis and osteoarthritis. These age-related disease causes a loss of independence and heavy economic burden. I have been working on aging research in Japan and United States with an ultimate goal of extending human healthy life by the development of effective and innovative healthcare technologies for age-related diseases, especially osteoarthritis.
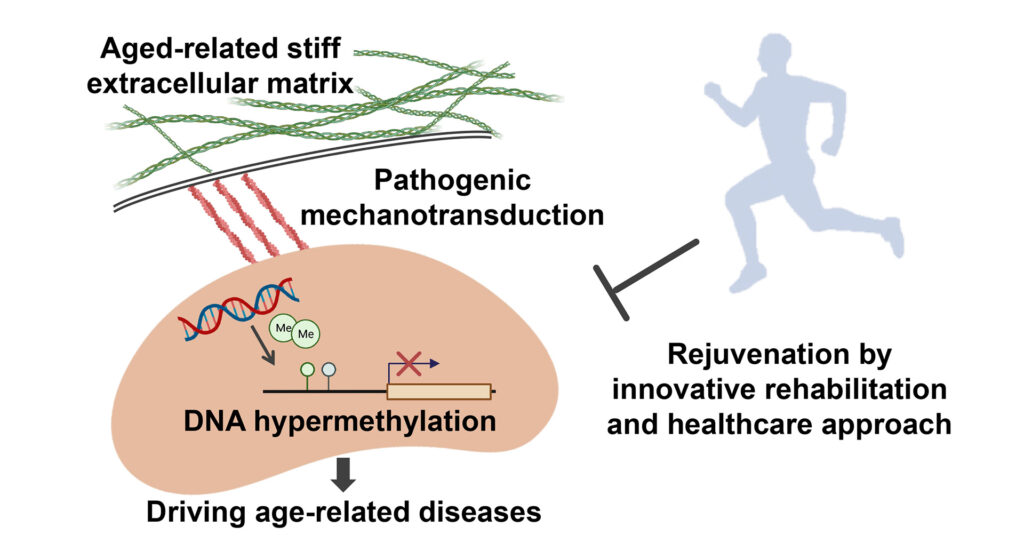
Accumulated evidence has shown that matrix stiffening is a quintessential feature of aged tissue including articular cartilage. Although it is recognized that matrix-derived forces influence chondrocytes health, the epigenetic mechanisms underlying age-related matrix alterations on the maintenance of the cellular health and function over time have remained elusive. Using integrated approach of bioinformatics with series of genetic and pharmacologic manipulations, I discovered that age-related alterations in matrix biophysical properties initiate pathogenic mechanotransductive cascades that induce α-Klotho promoter hypermethylation in chondrocytes and compromise articular cartilage integrity (Fig; Iijima H, OARSI 2020 Young Investigator Award; Iijima H, Nature Communications, Major revision). In the light of these data, we applied for a US patent (Methods and materials for treating osteoarthritis; US Patent App 2021).
With this in mind, I recently proposed new interdisciplinary research field, “Rejuvenative Rehabilitation”, with the goal of the development of innovative anti-aging therapeutics which maximize physical function using the principal of rehabilitation medicine. As a first step to achieve this new field, our proof-of-concept trial demonstrated that rehabilitative exercise serves anti-aging effects and restores a youthful phenotype of chondrocytes. I believe that the establishment of the “Rejuvenative Rehabilitation” accelerates our challenging to human aging.


Representative papers
Iijima H, et al. Age-related increase in matrix stiffness downregulates α-Klotho in chondrocytes and induces cartilage degeneration. bioRxiv 2021 (Nature Communications, major revision).
Iijima H, et al. Meta-analysis integrated with multi-omics data analysis to elucidate pathogenic mechanisms of age-related knee osteoarthritis in mice. J Gerontol A Biol Sci Med Sci 2022;glab386.
Research URL
Schoen Adams Research Institute at Spaulding Rehabilitation Hirotaka Iijima profile (constantcontact.com)
researchmap https://researchmap.jp/iijimahirotaka
Google Scholar https://scholar.google.com/citations?user=XAQHfdwAAAAJ
Global issues to be solved through this project
Investigation of epigenetic regulatory mechanism driving age-related diseases
Aging is a progressive degenerative process that results in an increased risk of chronic diseases. Inadequate understanding of cellular and molecular mechanism driving the age-related diseases impedes the development of therapeutics to expand healthy lifespan. This study will explore genome-wide epigenetic regulation of cellular aging by age-related matrix stiffening. To achieve this, we will implement an integrated approach of tissue engineering, genomics, and systems biology to elucidate molecular landscape of age-related diseases. More specifically, first, we will use in vitro models engineered to mimic the biophysical properties of young and aged articular cartilage, and we will evaluate the effect of matrix stiffness on genome-wide epigenetic reprogramming. We will then use mathematical model to explore methylation regulatory mechanism. Successful completement of this study provides insight into aging biological process and development of effective and innovative anti-aging therapeutics.
Interview
News
-
お知らせ
A paper by T-GEx Alumni Dr. IIJIMA Hirotaka (Harvard University) published in nature communications has been selected for the UJA Best Paper Award.
-
お知らせ
T-GEx Alumni Dr. Hirotaka IIJIMA (Harvard University) has been appointed as a member of the Editorial Board of The Journal of Physiology, one of six editorial fellows from around the world.
-
お知らせ
A paper by T-GEx Alumni Dr. IIJIMA Hirotaka (Harvard University) has been published in The Journal of Physiology.
-
お知らせ
A paper by T-GEx Alumni Dr. IIJIMA Hirotaka (Harvard University) has been published in Aging Cell.
-
お知らせ
T-GEx Fellow Dr.IIJIMA Hirotaka, Nagoya University, won the best poster award at the 35th Annual Meeting of the Japanese Society of Cartilage and Metabolism.
-
お知らせ | Activity Reports
The research result of T-GEx Fellow Dr. IIJIMA Hirotaka, Nagoya University, was published in Iyaku Keizaisha Publishing ” Iyaku Keizai “.
-
お知らせ
Dr. IIJIMA Hirotaka (Nagoya University) published his research results in Nature Communications and a news article was released by Harvard Medical School with his own comments.
-
お知らせ
T-GEx Fellow Dr. IIJIMA Hirotaka, Nagoya University, has made a big impact on the world by releasing his research results at the same time as Nagoya University and Harvard University.
-
お知らせ
The 2nd Kick-off meeting for AY2022 was held.
-
お知らせ
The name was changed to T-GEx Fellow and T-GEx Associate.
-
お知らせ
Academic Mentor for FY2022 T-GEx Fellows has been decided.
-
お知らせ
The 1st kick-off meeting for AY2022 T-GEx Fellows was held.
-
お知らせ
The 6 T-GEx Fellows were selected for AY2022 T-GEx program.